TA in dialogue: Vaccines and Gene Drives against Malaria
-
Type of event:
Panel discussion (TA in dialogue)
-
Venue:
Deutscher Bundestag, Platz der Republik 1, 11011 Berlin, Foyer des Paul-Löbe-Hauses
-
Date:
25.04.2022
-
Organiser:
TAB and Committee for Education, Research and Technology Assessment of the German Bundestag
-
Time:
17.00 to 18.30 (on-site until 19.30)
-
Broadcast:
Recording of the event on bundestag.de (only in German) or on the YouTube-Channel of the Bundestag (with autogenerated subtitles)
- Links:
What was it about?
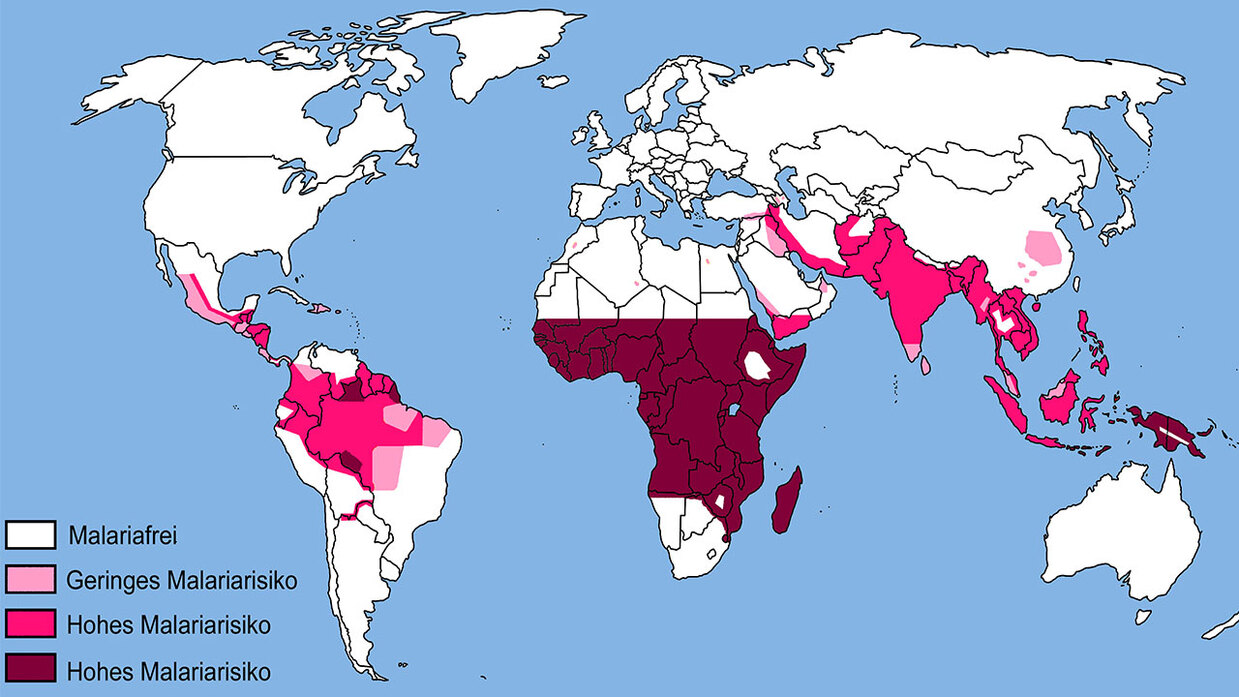
Malaria remains one of the most serious infectious diseases. Approximately 240 million people become infected each year, and more than 600,000 die from it - primarily in sub-Saharan Africa, with nearly 80% of victims being children under the age of 5. Although the WHO recommended a malaria vaccine for young children for the first time at the end of 2021, and it is hoped that four vaccinations will reduce child deaths by 30%. Nevertheless, there is widespread agreement that further control measures must be developed in order to substantially and permanently reduce and eradicate malaria in sub-Saharan Africa in the long term.
On the occasion of World Malaria Day on April 25, a moderated panel discussion presented both the status of vaccine development and the entirely new gene drive approach to combat the malaria-transmitting Anopheles mosquito. Invited experts and audience had the opportunity to discuss the potentials and challenges of these two technological approaches to malaria control. Thematically, the event tied in with two studies conducted by the Office of Technology Assessment at the German Bundestag (TAB) on behalf of the Committee on Education, Research and Technology Assessment: firstly, the project "Medicines for Africa" and, secondly, the ongoing project "Gene Drives - Technologies for Spreading Genetic Modifications in Populations", which analyzes the potentials and challenges of this complex biotechnological innovation.
The panel discussion (in German) addressed and questioned the scientific and technological possibilities with regard to their prospects for realization in the face of existing hurdles and obstacles of a socio-economic and regulatory nature.
The following questions guided the discussion:
- What potentials for malaria control are offered by vaccinations on the one hand and gene drive systems against malaria mosquitoes on the other? What challenges do these approaches face?
- How are both approaches regulated and approved? Which actors are responsible in each case? What criteria are used to decide on their use?
- What next steps are needed to further reduce malaria? What role can the two approaches play in an overall strategy for future malaria control?
Programme
| 17.00 h |
Greeting
Opening
Moderation of the panel discussion
|
| 5.10 p.m. |
Introduction to the topic
|
| 17.15 h |
Malaria as a global health problem
|
| 5:20 p.m. |
Status of malaria vaccine development
|
| 17.25 hrs. |
TARGET Malaria: Genetically Modified Mosquitoes and Gene Drives
|
| 5:30 p.m. |
Discussion on the podium with further experts
|
| 5:55 p.m. |
Discussion with the audience on site and online
|
| 6:25 p.m. | Summary of the discussion |
| 18.30 | Get-together / end of the broadcast on parliamentary television |
| 7.30 p.m. | End of the event at PLH |
Programme (PDF, in German only)
Digitally, the panel discussion could be followed live on Parliament TV and there was the possibility to contribute questions or comments to the discussion online via adhocracy+.
A summary review of the panel discussion can be found on the website of the German Bundestag.
