Bacteriophages in medicine, agriculture and food industry – application perspectives, regulatory and innovation issues
- Project team:
Arnold Sauter (Project Manager),
Harald König - Thematic area:
- Topic initiative:
Committee on Health, Committee on Food and Agriculture and Committee on Education, Research and Technology Assessment
- Analytical approach:
Innovation analysis
- Startdate:
2021
- Enddate:
2023
The final report was approved by the Committee for Education, Research and Technology Assessment on 05 July 2023 and and published in German on 18 July 2023. Our policy brief TAB-Fokus in English was released 05.09.2023.
Bacteriophages offer opportunities in the fight against multi-resistant pathogens
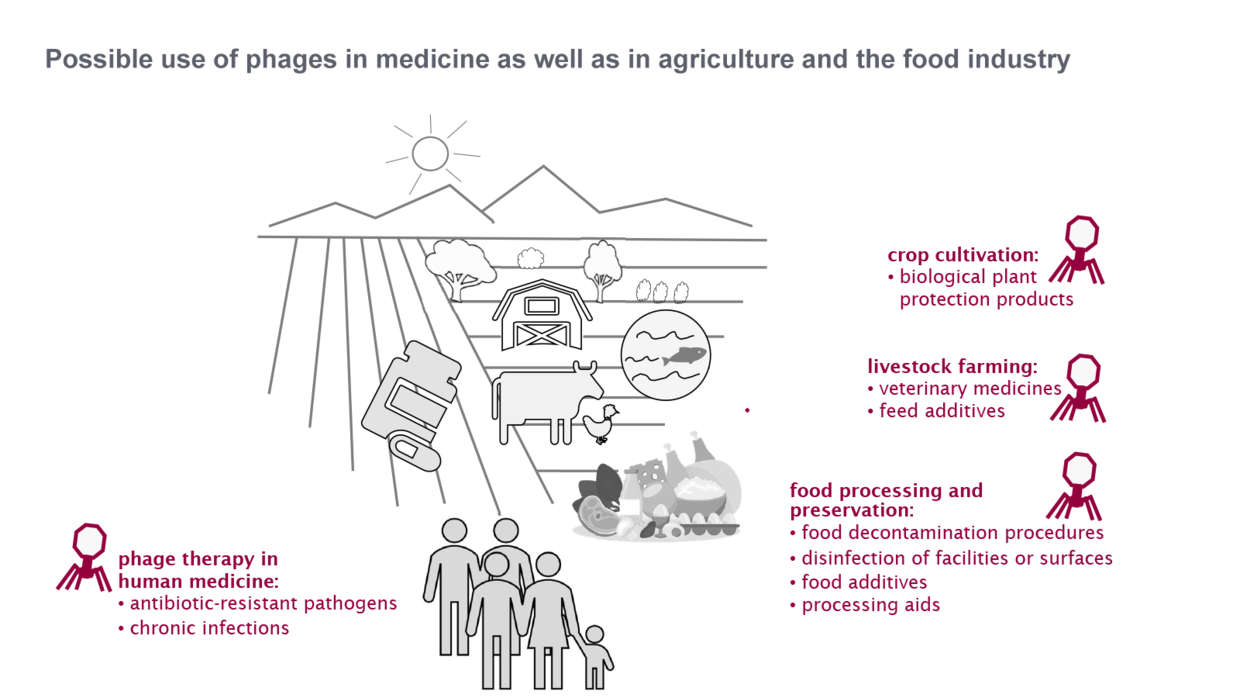
Antibiotic resistance is a global problem for human, animal and environmental health (One Health) and poses a major challenge for the German health system. Bacteriophages appear to be a relevant option to combat antibiotic-resistant bacteria in human medicine, crop cultivation, animal husbandry or food processing and preservation.
sprungmarken_marker_3892
What is involved
Bacteriophages (or phages for short) are viruses that can specifically attack and destroy certain bacteria. They are considered the most abundant biological entities on earth and, together with bacteria, are found in all habitats – including in animals and humans (with large numbers especially in their intestinal tract). Phages were discovered over 100 years ago and have been investigated and used as an option to fight bacterial infections – especially in humans, but also in animals and plants. In some countries of the former Soviet Union or Poland, phages have been used continuously as approved drugs or for personalised or experimental treatments of chronic and antibiotic-resistant infections in human medicine. In contrast, phages were hardly used for medical purposes in western industrialised countries for a long time after antibiotics became available in the 1940s.
Particularly in view of the worldwide growing problem of antibiotic resistance for human, animal and environmental health, phages are once again being discussed as a relevant option for combating pathogenic bacteria or bacterial infections in medicine as well as in agriculture and the food industry. Hence, approximately from the beginning of the 2000s, phages were taken into consideration again by researchers, physicians, patients and some young biotechnology companies for the treatment of infections that respond poorly or even not at all to antibiotics. In 2019, in Germany alone, more than 45,000 people died in association with antibiotic-resistant infections, i. e. on average one person every 12 minutes. In western industrialised countries, however, there is no phage preparation yet that is approved as a drug. And currently, phages can only be used under exceptional conditions in individual cases when available drugs have failed. Also, bacteriophages are increasingly being investigated for use in agriculture and the food industry. In these sectors, some commercial preparations are available or usable – but mainly outside the EU.
However, the special properties of phages compared to synthetic or chemical substances pose particular challenges for both the development and approval of effective drugs and commercial products to fight bacteria along the food chain. In order to better explore the existing multifaceted potential of phages in these areas and to be able to use it to a greater extent in the future, legal and innovation policy issues would have to be addressed in addition to scientific and technological questions.
Need for action and options
In particular, the ability to kill antibiotic-resistant bacteria and the possibility to overcome emerging bacterial phage resistance relatively easily and quickly make phage-based products a relevant option in the fight against the problems and challenges posed by antibiotic resistance to human, animal and environmental health (One Health). Thus there is a need to further explore and exploit the potential of this option.
Under the current framework conditions, however, it seems unlikely that the use of phages for phage therapy in human medicine or for applications in agriculture and the food industry will become established in the EU or in Germany better or on a larger scale than it has been so far.
This would require further development of the legal framework towards greater precision and flexibility, more comprehensive funding of research and development activities and possibly the creation of economic incentive structures.
Phage therapy in human medicine
Without marketing authorisation as a drug, phages can only be used in specific cases when no effective drugs are available. In Germany, such treatments have been carried out and documented in a few clinics. Although there is still a lack of evidence of efficacy from larger clinical trials in compliance with modern standards, clinical experience to date (especially from case studies of individual patients) suggests that phages may be an important option, particularly for the treatment of antibiotic-resistant infections - especially if phage preparations are tailored to the respective pathogens on a patient-specific basis.
In addition to scientific and technical issues that can be addressed by promoting a diverse R&D landscape and specific research programmes, there are also regulatory challenges to the successful development of phage therapy.
These are based on an inflexible regulatory framework in the EU that is largely geared towards invariable combinations of active pharmaceutical ingredients and standard therapies. On the other hand, the R&D costs for the most promising (personalised) phage therapies with a particular potential benefits, i.e. for infections that are difficult or impossible to treat conventionally with antibiotics, would have to be recouped by treating relatively small groups of patients.
Adjustments to the European regulatory framework and economic incentive schemes for new antimicrobial drugs are therefore likely to be needed to encourage or enable a broader and more diverse development of marketing approved phage products than has been the case to date.
However, the necessary adjustments at EU level could be lengthy or even fail. Alternatively, possible regulatory adjustments to the authorisation could prove to be too inflexible to also enable forms of treatment for which new phages would have to be isolated on a patient-specific basis. In parallel, it therefore seems necessary to design possible exemptions under EU law in Germany in such a way that phage therapies in special cases of need become accessible to a larger number of patients than it has been the case up to now. In particular, a practice-oriented adaptation along the lines of the Belgian model could make an important contribution to facilitating the treatment of individual patients with phage preparations prepared individually in specialised pharmacies (magistral phage preparations).
Phage applications in agriculture and the food industry
In the EU and Germany, phage preparations can only be used as processing aids in food processing without labelling and authorisation. There are no reliable figures on actual use. Outside the EU, some commercial phage-based products are used, covering a wide range of applications (e.g. control of certain plant diseases, surface decontamination of animal food). However, under the current framework conditions, it seems unrealistic that also in the EU phage products will become more widely used in agricultural and industrial practice in the foreseeable future. Yet bacteriophages should be considered and further developed as an option, especially with regard to foodborne infections and antibiotic resistance.
In addition to the scientific and technical problems that need to be solved with regard to the development of phage preparations for practical use, the legal framework, which is still mostly geared towards single, defined chemicals, is considered to be a major obstacle. The resulting requirements for the approval of phage products are often unclear and pose major challenges, especially for small and medium-sized companies, which are currently the main players in the development of commercial applications.
Policy initiatives or support are therefore needed in particular to review the regulation in all areas of application and, if necessary, to develop it further by adding specific guidance on phages. Clarifying the conditions of use could enable multiple applications both in agricultural production (veterinary and plant protection products) and in post-harvest food processing and preservation.
In addition, the promotion of longer-term research initiatives and networking activities - involving developing research institutions, companies and users - could contribute to capacity and competence building. Such initiatives should not least address safety research issues such as the potential spread of phage resistance as a result of the widespread use of bacteriophages.
Download Policy Brief and full report
|
TAB-Fokus no. 43 Bacteriophages in medicine, agriculture and food industry – application perspectives, regulatory and innovation issues Provides a compact four-page overview of the contents and results of the full report in German. |
|

|
TAB-Arbeitsbericht Nr. 206 (in German only Bakteriophagen in Medizin, Land- und Lebensmittelwirtschaft – Anwendungsperspektiven, Innovations- und Regulierungsfragen. Innovationsanalyse (PDF) The TAB report provides a comprehensive overview of the state of development and potential applications of bacteriophages in medicine, agriculture and the food industry, and analyses the different regulatory frameworks. From this, scientific-technical, economic, innovation-policy and legal challenges and options for action to promote and further develop the use of phage are derived and described in detail. The TAB report thus provides an up-to-date and well-founded information base on this important topic of research, health, agricultural and environmental policy. |
In the Bundestag
(only in German)
- Vorgang - Bericht, Gutachten, Programm im Dokumentations- und Informationssystem für Parlamentsmaterialien (DIP)
↵
In the media
(only in German)
- aerzteblatt.de (26.01.2024) Eine Zukunft mit Phagen? Das Potenzial von Phagen bei der Bekämpfung von Antibiotikaresistenzen sollte stärker genutzt werden. Zu diesem Schluss kommen Berichte aus Deutschland und dem Vereinigten Königreich. Ein aktueller Gesetzgebungsprozess wird entscheiden, ob Richtlinien ihre Nutzung in der EU weiter behindern werden.
- nd-aktuell.de (24.08.2023) Virus kontra Bakterium. Phagen sind vielversprechend im Kampf gegen antibiotikaresistente Keime, aber noch ist die Anwendung weit entfernt.
- taz.de (21.08.2023) Phagen als Alternative zu Antibiotika: Resistent gegen Resistenzen. Antibiotikaresistenzen fordern immer mehr Todesopfer. Hoffnung macht die Phagentherapie – eine im Westen fast unbekannte Heilmethode.
- vdi-nachrichten.com (+) (09.08.2023) Mit Bakteriophagen gegen Krankenhauskeime: Chancen und Hürden im Überblick.
- spektrum.de (27.07.2023) Blasenentzündung: Mit Phagen gegen resistente Krankheitserreger.
- fr.de (27.07.2023) Behandlung mit Phagen könnte eine Alternative zu Antibiotika sein.
Noch dürfen Phagen – spezielle Viren – in der EU nicht eingesetzt werden. Fachleute plädieren dafür, die geltenden Richtlinien zu ändern. - ardaudiothek.de (23.07.2023) Viren gegen Bakterien.
Mit Harald König zur Bakteriophagen-Studie, ab Minute 12:20 - dw.com (21.07.2023) Bakteriophagen vs. Antibiotika: Mit Viren gegen Resistenzen
- sueddeutsche.de (20.07.2023) Antibiotikaresistenzen: Kampf der Winzlinge.
Seit Langem wird an Bakteriophagen geforscht: Viren, die Bakterien bekämpfen können, wenn Antibiotika versagen. Doch eingesetzt werden sie nur in Ausnahmefällen. Ließe sich das ändern? - br.de (20.07.2023) Phagen gegen Antibiotikaresistenzen: Mehr Forschung gefordert.
Im Kampf gegen Antibiotikaresistenzen hat die Medizin ein altes Heilmittel neu entdeckt: die Bakteriophagen, kurz Phagen. Experten fordern jetzt mehr Forschung dazu. Das Besondere an Bakteriophagen und warum ihr Einsatz noch schwierig ist. - br.de (19.7. 2023/14.08.2023) Bakteriophagen: Bundestagsexperten empfehlen Förderung. Audiobeitrag (14:08) u.a. mit TAB-Berichtautor Harald König.
- sciencemediacenter.de (18.07.2023) Bakteriophagen als Alternative zu Antibiotika.
Das SMC hat Forschende gebeten, die Hürden und Anwendungsperspektiven von Phagen in der Medizin sowie die im TAB-Bericht vorgeschlagenen Maßnahmen einzuschätzen. - heise.de (18.07.2023) Empfehlung von Expertenrat: Heilende Phagen schneller in die Praxis bringen.